This article was originally posted on the blog Dinner Table Science were I was guest blogging – please visit and follow Rachel’s blog, it is great!
Life, as we know it, would not have been possible without the carbon atom. The scientific discipline dealing with all the molecular relationships that the carbon atom is getting itself into – organic chemistry – is essential for understanding how living organisms function and evolve. We take it for granted that organic chemistry is flourishing on Earth, because it is teeming of life, but only in the last few decades have we come to realize that such processes also are common far beyond our own planet – in the depths of space. No form of extraterrestrial life has been discovered so far, but a surprisingly rich variety of organic compounds has been found within the Solar System, as well as in the interstellar medium. None of these organic molecules have a biological origin but some may, or may not, have been prebiotic, i.e., involved in the processes that eventually led to the emergence of life on our planet.
Organic molecules are found in many different Solar System objects or space environments, but here we will focus on two of them – a type of meteorite called a carbonaceous chondrite, and the interstellar medium. After reviewing what we know about these organic substances, we will also discuss the processes believed to have created them.

The Murchison meteorite is a carbonaceous chondrite. It is one of our most important sources of information on organics from space due to its large mass (more than 100 kg) and the fact that it was recovered right after it fell, and has suffered a minimum of terrestrial contamination. This picture shows a piece of Murchison at the The National Museum of Natural History (Washington). Original image: http://en.wikipedia.org/wiki/File:Murchison_crop.jpg
Carbonaceous chondrites
Most of the meteorites that impact Earth, almost 90%, are so-called chondrites. They got their name because they are rich in chondrules, a type of millimeter-sized grain formed in huge numbers 4.57 billion years ago during the earliest phase of Solar System history. They still contain these ancient particles because the chondritic meteorite parent bodies never melted (these 10-100 km parent bodies later broke up in devastating collisions, and the chondritic meteorites are tiny fragments from such collisions). This distinguishes the chondritic meteorites from achondritic meteorites (8% of the falls) and iron meteorites (2% of the falls) that both originate from parent bodies that once were heated by radioactive decay to the point that they actually melted and differentiated, e.g. separated into a rocky mantle and a metallic core. Such melting erased all memory of previous history, and that is why chondritic meteorites are so valuable – they can tell us about the time before the formation of the parent bodies (most of which eventually were merging to form the planets).
Most chondritic meteorites are so-called ordinary chondrites that are related to the rocky S-type asteroids in the inner main asteroid belt. This region was generally too warm to allow molecular compounds with a low boiling point to condense into solids. However, some of the meteorites are carbonaceous chondrites, believed to be related to the more distant C-type asteroids from the outer main asteroid belt. They formed in conditions sufficiently cold to allow condensation of very volatile species – they are therefore rich in water (up to 20% by mass) and they also contain organic molecules that are rare or absent in the inner Solar System.
The carbonaceous chondrites got their name because they are unusually dark – they look like charcoal. The name is actually misleading, because the dark color is not due to carbon, but due to iron, an atom that is very efficient in absorbing light. In ordinary chondrites, the iron is gathered into small metallic particles mixed with the chondrules (we say that the iron is reduced), leaving the rocky material virtually iron-free and thus fairly bright in color. In carbonaceous chondrites, the iron is finely distributed throughout the rocky material on an atomic level (we say that the iron is oxidized), which makes the entire rock an efficient light absorber, thus being dark.
The typical carbonaceous chondrite contains only about 2-5% carbon by mass. However, this organic material is amazing, particularly the 25% that is referred to as “extractable organic matter” that is either liquid or solid at room temperature. In decreasing order of abundance the extractable organic matter consists of the following cocktail, of which a selection is described below – carboxylic acids, sulfonic acids, amino acids, sugars, urea, aliphatic and aromatic hydrocarbons, ketones, ammonia, alcohols, purines and pyrimidines. The remaining 75% is called “macromolecular material” and is a solid substance with an extremely complex composition. In some cases, the organics in meteorites is partially terrestrial contaminations. However, the risk of contamination is low if meteorites are recovered directly after a fall, and in most cases the meteoritic organics have such unusual concentrations of the isotopes carbon-13, nitrogen-15 and hydrogen-2 (deuterium), that a terrestrial origin can be excluded. Thus, the compounds discussed below are definitively from space.
In order to understand how the macromolecular material looks like, we need to know how carbon atoms interact with their own kind and with other atoms. A carbon atom can bind itself to up to four other atoms simultaneously. Often, carbon atoms form linear chains of various length. A given carbon atom within the chain then connects with two of its carbon neighbors, meaning that it can afford to connect also with two other atoms, typically hydrogen (but sometimes oxygen, nitrogen or other atoms). The carbon atom sitting at the end of a chain only connects with one other carbon, allowing it to attach three hydrogen atoms to it. These chain-like structures are called aliphatic hydrocarbons. However, it is also common that six (and sometimes five) carbon atoms form a ring. Here, a given carbon atom uses two of its connections to bind to the first of its carbon neighbors, and one for the second, leaving space for a single foreign atom (often hydrogen) to attach itself externally to the ring. Such ring-like structures are called aromatic hydrocarbons. Rings can also attach to each other, so that two carbon atoms simultaneously are members of two rings. Molecules that contain several such rings are called polycyclic aromatic hydrocarbons (or PAHs).
The macromolecular material in carbonaceous chondrites has proven to be a gigantic web, where aromatic rings use aliphatic chains to connect to each other. Often there are single rings that have replaced one or several of their external hydrogen atoms with an aliphatic chain – typically with 2-4 carbon atom links – in order to connect to another aromatic ring located farther away. However, it is not unusual that two, three or four rings form a little aromatic island, that connects itself to other islands through the aliphatic bridges. The fraction of macromolecular carbon that is aromatic is about 60-70% for Murchison, about 70-80% for Orgueil and almost 100% for Tagish Lake (these are three different carbonaceous meteorites). A typical elemental composition of the macromolecular material is about 70 hydrogen atoms (H), 12 oxygen atoms (O), three nitrogen atoms (N) and two sulphur atoms (S), for every 100 carbon atoms (C).
The extractable organic matter is dominated by carboxylic acids – these are basically aliphatic chains where one carbon atom at the end of the chain has replaced two of its hydrogen atoms with a single oxygen atom, and replaced the last hydrogen atom with a hydroxyle group consisting of an oxygen atom with a hydrogen attached to it (thus there is a COOH group). If both ends of the chain has COOH groups, the molecule is called a dicarboxylic acid. Examples include formic acid (HCOOH) used by ants as a venom, acetic acid (CH3COOH) used in cooking in diluted from under the name vinegar, butyric acid (C3H7COOH) that gives rancid butter its unpleasant smell, and valeric acid (C4H9COOH) that is named after the valerian herb (Valeriana officinalis) that produces this molecule. They are all found in carbonaceous meteorites, that often contain carboxylic acids with up to ten carbon atoms.
If a carboxylic acid has one of its hydrogen atoms in the aliphatic chain replaced by the amino group NH2, it is called an amino acid. Amino acids are fundamental to life, because they are the building blocks of proteins. Humans and other animals need to eat proteins, and the body break them down into their amino acid constituents, that are then used by cells to build other proteins (a process called translation) that we need to function. To find amino acids in meteorites is extremely fascinating, just because they play such a central role in the chemistry of living organisms.
More than 70 different amino acids have been identified in carbonaceous meteorites. Some of these meteorites, like Murchison, Orgueil and Ivuna are rather rich in amino acids. Others, like Tagish Lake, have extremely low abundances of amino acids. Murchison contains eight of the protein amino acids, eleven that are biologically common, and several others that are not used by terrestrial organisms. The five most common, in decreasing order of abundance, is glycine (NH2[CH2]COOH), alpha-aminoisobutyric acid (NH2[C3H6]COOH), D-alanine and beta-alanine (NH2[C2H4]COOH), and isovaline (NH2[C4H8]COOH), where the chemical formulae have been written to highlight the amino and carboxyl groups. Here, the prefixes “alpha” and “beta” is a way to tell which carbon atom the amino group is attached to.
Most amino acids have so-called chirality, which means that there are two variants of each molecule (called enantiomers), that have the same chemical composition but geometrically are mirror images of each other. They are distinguished through the prefixes L and D such as in L-alanine and D-alanine. All proteins built by translation contain L enantiomers. In carbonaceous chondrites both enantiomers appear to be equally common, although some controversial studies show that there may be a slight L-excess for certain amino acids like alanine, proline and leucine.
Aromatic hydrocarbons are not only found in the macromolecular material, but also in the extractable organic material. The most common compounds are the PAHs fluoranthene and pyrene. They both have the formula C16H10 but are structurally different – flouranthene consists of three standard rings with six carbon atoms, joined by a ring containing just five carbon atoms, while pyrene is made of four standard rings.
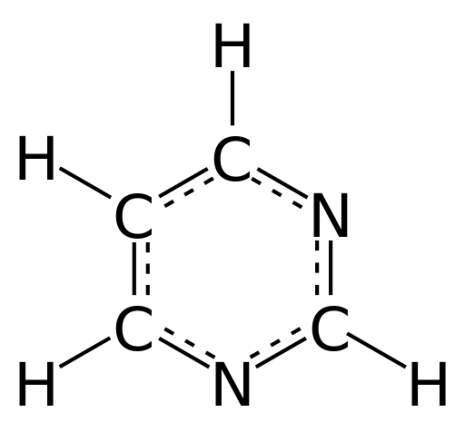
The structural formula of pyrimidine, showing how carbon (C) and nitrogen (N) atoms form a ring, to which a number of hydrogen (H) atoms are attached. Original image: http://en.wikipedia.org/wiki/File:Pyrimidine_2D_aromatic_full.svg
The really interesting thing starts when the carbon atoms in aromatic rings are replaced by nitrogen. If the starting point is a single six-atom ring (called benzene if all atoms are carbon), and two specific carbon atoms are exchanged with nitrogen, a substance called pyrimidine (C4H4N2) is formed. By replacing hydrogen atoms by amino groups or oxygen atoms, a variety of molecules can be formed, including the nucleobases cytosine (C), thymine (T) and uracil (U), that are basic building blocks in DNA and RNA. The pyrimidine uracil has been found in carbonaceous chondrites.
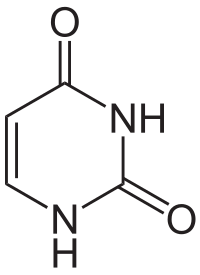
The structural formula of uracil. The aromatic ring is here somewhat abstract since corners are meant to indicate the location of a carbon atom (sometimes with a hydrogen atom attached to it), while only nitrogen and oxygen atoms, with associated hydrogen atoms, are shown explicitly. Original image: http://en.wikipedia.org/wiki/File:Uracil.svg
If the starting point is a six-atom ring joined to a five-atom ring, and two specific carbon atoms in each ring are replaced with nitrogen, a compound called purine (C5H4N4) is obtained. As before, the replacement of hydrogen atoms with amino groups and oxygen atoms gives rise to a variety of molecules, including two other nucleobases that are found in DNA – adenine (A) and guanine (G). Both adenine and guanine has been found in carbonaceous chondrites, along with other purines, such as xanthine and hypoxanthine.
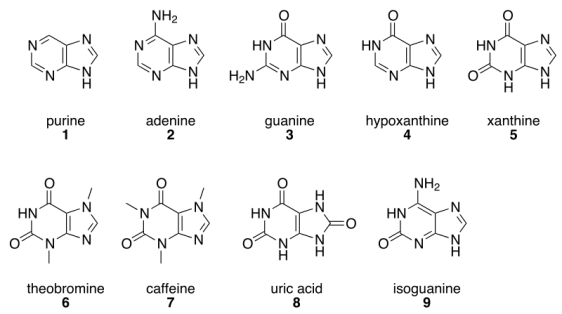
The structural formula of various kinds of purines. Adenine, guanine, hypoxanthine and xanthine have been found in carbonaceous chondrites. Original image: http://en.wikipedia.org/wiki/File:Purines.svg
It is therefore clear that carbonaceous chondrites contain a variety of fairly complex organic molecules. Comet nuclei are likely to be as rich or even richer in such compounds, although we know much less about comets than meteorites since actual samples of comet material is restricted to very small amounts, collected by the Stardust spacecraft in the coma of Comet 81P/Wild 2 and brought back to Earth. Both carbonaceous chondrites and comets bombarded the young Earth, thereby bringing organic substances to the inner region of the Solar System, where such compounds initially may have been rare. The possibility that these organics were involved in the formation of life on Earth is a fascinating thought. But what is the origin of these compounds – where did they form and how? To answer that question, we must leave the Solar System and head out into interstellar space.
The interstellar medium
The space between the stars in our galaxy, the Milky Way, is not empty – about 90% of the galactic mass is bound in stars, while the rest forms an extremely thin mixture of gas and solid dust particles that fill the space between the stars – the interstellar medium. About 98-99% of the mass is hydrogen and helium, while the remaining fraction is shared between all heavier elements. Among these, oxygen is the most common element by number, followed by carbon, neon, and nitrogen. These elements mainly exist as unbound atoms in the gas phase, while the solid grains primarily consist of silicates and sulfides rich in oxygen, magnesium, silicon, iron and sulphur (these are the ten most common chemical elements in the universe, by number).
In places where the interstellar medium is particularly dense – the molecular clouds – atoms in the gas phase form small molecules, primarily molecular hydrogen (H2), carbon monoxide (CO), and molecular nitrogen (N2), but also water (H2O), carbon dioxide (CO2), ammonia (NH3), methane (CH4), and methanol (CH3OH). Over time, some of these gases will condense on top of the grains, thus forming mantles of ice that surround the rocky cores.
When such ice is exposed to ultraviolet radiation from nearby stars, it gets damaged. Molecules are cut into small pieces called radicals. These are extremely reactive, but due to the extreme cold (typically 10K or -260C) the radicals have little mobility and do not manage to get in physical contact with each other. Over time, large deposits of radicals are built up within the ice. Only a small amount of heating, perhaps due to rare collisions between icy grains, is sufficient to trigger an explosive chain reaction, were radicals unite to form a variety of complex organic molecules. Many of these leave the grain surfaces and can be observed as free molecules in the interstellar gas, while others remain on the grain surfaces.
This process is at least partially responsible for the rich variety of organic molecules that has been observed in interstellar space. Observations are made with radio telescopes, that pick up the long-wavelength radiation that the molecules emit when they change their rotation or vibration rates. More than 150 molecular compounds have been identified in the interstellar medium, of which a third contain six atoms or more. Some large molecules that have been identified include propylene (CH3CHCH2), methyltriacetylene (CH3C6H), vinyl alcohol (C2H3OH), acetic acid (CH3COOH), ethylene glycol (HOCH2CH2OH), cyanopentaacetylene (HC11N), and acetamide (CH3CONH2).
The processes taking place in the interstellar medium can be reproduced in laboratories. A mixture of ice (for example, water, carbon monoxide, carbon dioxide, ammonia, and methanol in proportions expected in interstellar ice) is deposited on a 10K substrate, and the mixture is irradiated by ultraviolet radiation and then slowly heated to trigger the chain reaction. An organic residue is thus formed, sometimes called “yellow stuff”. The material is particularly rich in carboxylic acids and hexamethylenetetramine (C6H12N4). In one particular experiment, no less than 16 amino acids where identified in this yellow stuff. These included glycine, alanine, sarcosine, valine and serine. In these samples both enantiomers of each amino acid were equally common, to within measurement uncertainties.
Stellar and planetary systems form when interstellar gas and dust collapse due to its self-gravity, in regions where the interstellar medium has become exceptionally dense. According to the “interstellar parent-body hypothesis” the organic compounds seen in carbonaceous chondrites are interstellar organics that survived the turmoil of Solar System formation. That is to say, these substances did not primarily form here, and at least a fraction of the material must have avoided heating to the point where they would have disintegrated into their atomic constituents. The material may have been processed or altered in various ways, but the key idea is that the carbonaceous chondrite parent bodies contained complex organics already when they formed. The very unusual isotopic composition that characterize these organics are often interpreted as evidence of an interstellar origin.
Although the carbonaceous chondrite parent bodies formed sufficiently late not to melt (perhaps 1-2 million years after differentiated bodies, at a time when the short-lived radioactive heat source aluminum-26 almost had vanished), they still experienced mild warming. The temperature was sufficiently high to melt ice within the parent body, and allowing liquid water to percolate through the granular interior. This has led to various levels of so-called aqueous alteration – characteristic changes of the mineralogical composition of the meteorite caused by liquid water. The presence of liquid water has also modified the composition of the organics. It appears increasingly unlikely that the organics we see today in carbonaceous chondrites were formed from scratch during aqueous alteration – they must have had a long and complex previous evolutionary history that started in interstellar space.
The exploration of organics in space has merely begun. The presence of complex organic molecules in distant asteroids and comet nuclei are strong reasons for performing sample return missions to such bodies. The answers to some of our most profound questions about our existence may lay buried within these ancient survivors of planetary formation – how did the Solar System form, what kind of material rained down on the young Earth, and is it possible that life got a jump start thanks to organics from space?
We just have to go and have a look.
Literature
Gilmour, I. (2003). Structural and isotopic analysis of organic matter in carbonaceous chondrites. Treatise on Geochemistry, 1, 269-290.
Herbst, E., van Dishoeck, E. F. (2009). Complex organic interstellar molecules. Annual Review of Astronomy and Astrophysics, 47, 427-480.
Muñoz Caro, G. M., Meierhenrich, U. J., Schutte, W. A., Barbier, B., Arcones Segovia, A., Rosenbauer, H., Thiemann, W. H.-P., Brack, A., Greenberg, J. M. (2002). Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 416, 403-406.
